 Worked on a whopper of a project, for Microsoft. My company was picked to help develop a “Solution Accelerator” for the Pharmaceuticals industry, providing a SharePoint implementation template for clinical trials, as well as some InfoPath data-gathering tools.
Worked on a whopper of a project, for Microsoft. My company was picked to help develop a “Solution Accelerator” for the Pharmaceuticals industry, providing a SharePoint implementation template for clinical trials, as well as some InfoPath data-gathering tools.
Clinical trials are enormously complex, regulated, and expensive. We focused on the protocol creation and approval process, which contains aspects of collaborative document authoring and workflow, regulatory submission and approval, etc. I had always thought enterprise software projects were big things, but taking a look at clinical trial protocols helped put that in perspective.
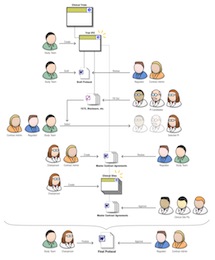
The challenge was to learn a lot very fast, in order to be able to add value. In about five weeks, we nailed down the realities of the process of protocol creation and submission, and I insisted we also focus on the context and goals of the different actors and stakeholders. I designed a nifty cast of characters, which we used in process diagrams to make the workflow come to life. They proved unexpectedly useful.
What happened is that the little sketch you see here helped everybody on the team stay on the same page in terms of user functionality. Developers working on a module could see the gal in the white lab coat and glasses using their module, and they made the interface precise and data-rich. UIs were markedly different, more verbose and action-oriented, for the suit-and-tie users.
I created countless flow diagrams, worked closely with developers to ensure all requirements were covered, and designed the SharePoint UI to host and unify it all. On a project of such scale and urgency, the difficulty was to not let user considerations get buried under the colossal weight of enormous and dense requirements. Tensions ran high, but we delivered, and the client was satisfied with the result.